
Not actual patient.
Study Results

Not actual patient.
PEDMARK showed it could help protect a person’s hearing after cisplatin in 2 different studies of people 1 month of age and older.
PEDMARK was studied around the world
In patients 1 month of age and older
SIOPEL Study
The International Childhood Liver Tumor Strategy Group (SIOPEL) studied patients between 1 month and 18 years of age receiving cisplatin-based cancer treatment for standard risk hepatoblastoma (solid tumor in the liver).
In all, 114 patients were tested for hearing loss: 61 in the PEDMARK + cisplatin group and 53 in the cisplatin-only group.
Percentage of patients with hearing loss (ITT population) in SIOPEL 6 (n=114)
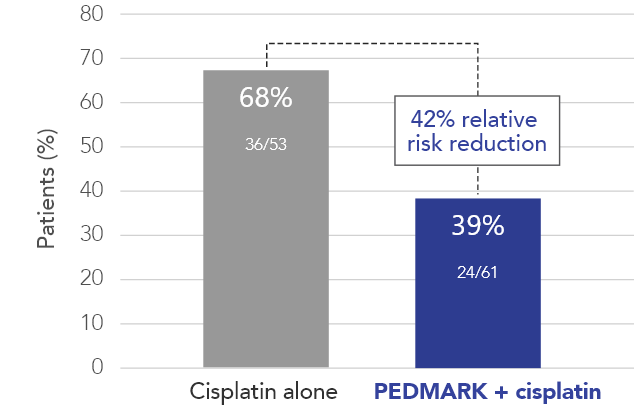
PEDMARK reduced hearing loss by 42% compared with cisplatin alone
Percentage of patients with hearing loss (ITT population) in COG ACCL0431 (n=77)
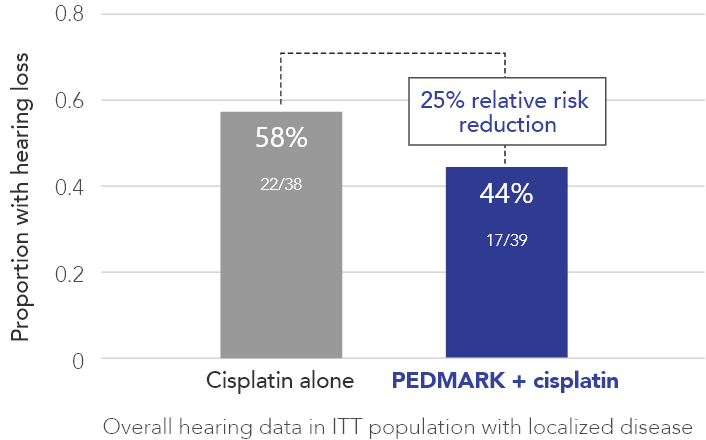
PEDMARK reduced hearing loss by 25% compared with cisplatin alone
COG Study
The Children’s Oncology Group (COG) studied patients between 1 year and 18 years of age with tumors that have not spread to other parts of the body receiving cisplatin-based cancer treatment. In all, 77 patients with tumors that have not spread to other parts of the body were tested for hearing loss: 39 in the PEDMARK + cisplatin group and 38 in the cisplatin-only group. All patients were on their first treatment for one of the following cancers:
- Germ cell tumors (tumors that form from cells that create either sperm or eggs)
- Hepatoblastoma (solid tumor of the liver)
- Medulloblastoma (a tumor that begins in the brain or spinal cord)
- Neuroblastoma (a tumor that forms in nerve cells)
- Osteosarcoma (bone tumor)
- Other cancers treated with cisplatin
How hearing loss was measured
In both studies, scientists measured hearing loss using pure-tone audiometry. This is a hearing test that shows the softest sound a person can hear at least 50% of the time.
The source for access support
Fennec HEARS™ is the single source for both financial and patient support. Call 1-833-7PEDMARK.


Get more information about PEDMARK
Find helpful tools and resources.
IMPORTANT SAFETY INFORMATION
- Do not allow your child to receive PEDMARK if they have had a severe allergic reaction to sodium thiosulfate or any of the other ingredients in PEDMARK.
- Allergic reactions can happen with PEDMARK and can be serious and life-threatening. Your healthcare provider will monitor your child for allergic reactions during the PEDMARK infusion. Your healthcare provider will stop the infusion and provide treatment if your child has an allergic reaction. If your child has an allergic reaction, your healthcare provider will give your child certain medicines before each PEDMARK infusion. Tell your healthcare provider right away if your child has any of these signs of an allergic reaction: rash, hives (raised bumps), chest tightness, wheezing, trouble breathing, swelling of the face, lips, tongue, or throat.
- Changes in salt and potassium levels in the blood are common with PEDMARK but can also be serious. Your healthcare provider will do blood tests to check your child’s sodium and potassium levels before starting and as needed during treatment with PEDMARK. Tell your healthcare provider right away if your child develops any of the following signs or symptoms: feeling tired or weak, feeling restless, muscle weakness, seizures.
- Nausea and vomiting are common with PEDMARK but can also be serious. Your healthcare provider will give your child medicines before each PEDMARK infusion to help prevent nausea and vomiting.
- The most common side effects of PEDMARK include decreased red blood cells (anemia).
- These are not all the possible side effects of PEDMARK. Call your child’s doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Fennec Pharmaceuticals, Inc. at 1-833-336-6321.
Please see full Prescribing Information, including Patient Information, for PEDMARK.
WHAT IS PEDMARK?
PEDMARK is a prescription medicine used to decrease the risk of hearing loss in children 1 month of age and older who are receiving cisplatin for solid tumors (cancer) that have not spread to other parts of the body.
It is not known if PEDMARK is safe and effective when given after cisplatin infusions longer than 6 hours.
It is not known if PEDMARK is safe and effective in children less than 1 month of age. PEDMARK is not recommended in children younger than 1 month of age.

